
José Manuel Torralba, IMDEA MATERIALS
They surround us in computers, bottles, packaging, furniture, cars, aeroplanes and even in most of the clothes we wear. Their low cost and apparent recyclability have made polymers (plastics) ubiquitous. However, they present two major problems.
The first is that only thermoplastics are recyclable and, even then, less than 10% are actually recycled. Moreover, after each recycling cycle, their polymer chains degrade, which limits their reuse. The rest ends up in landfills, rivers and oceans.
The second problem is that plastics are soft and degrade easily, forming microplastics and nanoparticles that end up in water, air and soil. They also reach our bodies and those of other living organisms. They are even transported by bees along with pollen.
Betting on biodegradability
The materials that were the protagonists of the 20th century have now turned out not to be “sustainable”. They are generating a major environmental problem and can also damage our health. The good news is that there is a solution.
Achieving it requires combining two elements: legislation that rewards the use of alternative materials, and major investment in R&D to develop plastics that are more recyclable and, above all, biodegradable.
In this direction, there are many possibilities. For example, searching for alternative polymers to those derived from petroleum, which currently account for the vast majority.
Disintegrating in salt
At the RIKEN Center for Emergent Matter Science, in collaboration with the University of Tokyo (Japan), researchers have developed a polymer (still at the research stage) that disintegrates when it comes into contact with salt.
This allows the material to dissolve in seawater within a few hours. In addition to being non-toxic and fire-resistant, it does not release carbon dioxide during degradation. Although it has not yet been commercialised, numerous companies, especially in the packaging sector, have already expressed interest.
These new polymers are as strong as those commonly used in industry. The difference is that, when they decompose naturally, their components are biodegraded by bacteria present in the environment and therefore do not accumulate or form microplastics. Similarly, salts present in soils also allow them to decompose on land.
Greedy bacteria
Meanwhile, a research group at the University of Kobe (Japan) has developed pyridinedicarboxylic acid (PDCA), a biological polymer capable of achieving the performance of some plastics such as PET, the most widely used material for bottling water and soft drinks.
Unlike PET, however, PDCA is fully biodegradable and is produced through synthesis using bacteria and enzymes. Among them is the bacterium Escherichia coli, fed with glucose to accelerate production.
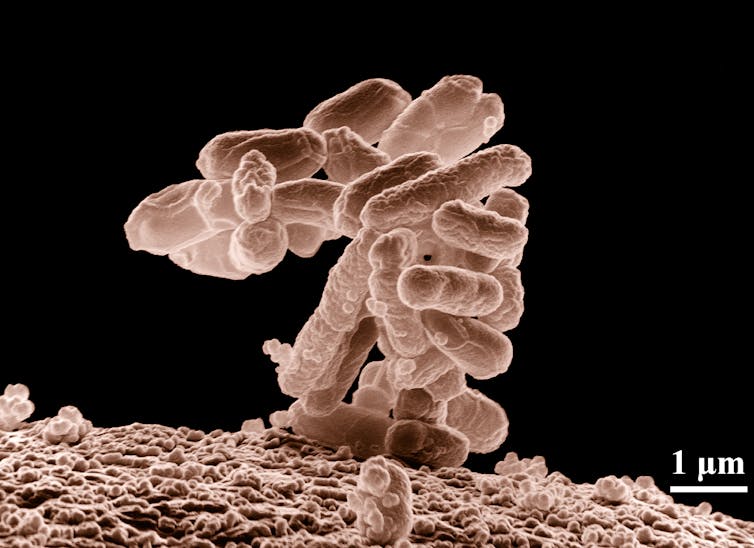
This bacterium and its appetite for glucose were also the focus of another recent study, published in Nature Chemistry, on how to generate biofuels naturally.
Materiales competitivos
In China, plastics derived from bamboo cellulose are being developed. According to a study published in Nature Communications, solvents are used to break down the hydrogen-bond network of bamboo cellulose. Subsequently, with molecular stimulation assisted by ethanol, the hydrogen bonds are reconstructed, resulting in a bioplastic with exceptional mechanical strength that can also be manufactured using conventional injection moulding technologies.
This material outperforms most commercial plastics and bioplastics in terms of mechanical and thermomechanical properties. In addition, it is fully biodegradable in soil within 50 days. The study also presents a technical and economic analysis demonstrating the material’s cost competitiveness, helping to bridge the gap between sustainability and industrial scalability.
Microplastics turned into graphene
At the same time, solutions for microplastics are beginning to emerge. At James Cook University in Australia, researchers have carried out a study in which microplastics are treated using a technique known as atmospheric pressure microwave plasma (APMP), in a microwave oven at atmospheric pressure, to transform them into graphene: a high value-added material.
The researchers claim they can convert 30 mg of microplastics into 5 mg of graphene in just one minute. This technology is much faster, operates at significantly lower temperatures and consumes less energy than more traditional methods such as pyrolysis or catalytic carbonisation.
As a result, the efficient transformation of polyethylene microplastics from discarded bottles into graphene is now a reality.
Political will and investment are needed
As we can see, the scientific field has reached sufficient maturity to address these problems with viable solutions.
However, political will is essential to launch R&D programmes that guide research groups, through adequate funding, towards making the dream of fully recyclable, biodegradable and non-polluting polymers a reality.
José Manuel Torralba, Full Professor at the Carlos III University of Madrid, IMDEA MATERIALS
This article was originally published in The Conversation. Read the original (content in Spanish).
